NEUROTOXINS:
- Treat dynamic lines and wrinkles (crow’s feet, frown lines) without surgery
- Quick injectable treatment with little to no downtime
- Long-lasting results with maintenance treatments
Injectable Wrinkle Treatment
Chances are, you’ve heard its name mentioned before — maybe even know someone who’s raved about its youthful results. Xeomin® and other botulinum toxin injectable treatments continue to be among the most popular aesthetic treatments for putting an end to dynamic facial lines and wrinkles. As a board certified plastic surgeon, Dr. Duncan often recommends Xeomin® and DAXXIFY™ to her Colorado patients who want to maintain a youthful appearance, but who do not yet need (or are not ready for) surgery to achieve their desired outcome.
Neurotoxins may be right for you if you are concerned about:
- Crow’s feet (at the corners of the eyes)
- Frown lines or glabellar lines (between the brows)
- Forehead wrinkles
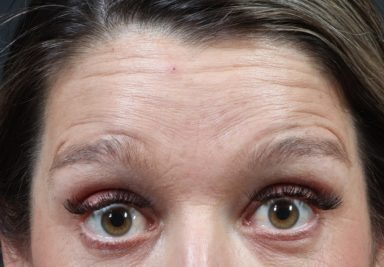
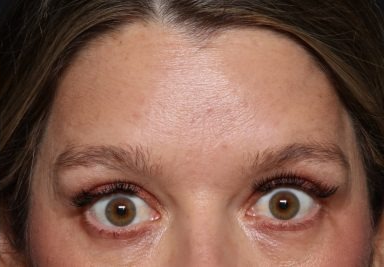
REAL PATIENT STORY
This 39 year old mom disliked her ‘mom face’ and sought Xeomin to help smooth out the dynamic lines of her forehead and glabella.
39 year old before & after injections neuromodulator (Xeomin) injections for dynamic lines of the forehead
Our Approach to Neurotoxins
Achieving results that look as natural as they do youthful requires the experience and eye of a trained physician. Our team takes the necessary time with each of our patients to ensure we understand their specific concerns and goals. We will make treatment recommendations based on each individual consultation and will personalize the injectable treatment to meet the patient’s unique needs.
What to Expect with Treatment
This treatment is performed in-office and requires no surgery or anesthesia. The treatment site(s) will be marked prior to injection, and the number of units required will be determined during your consultation with Dr. Duncan or our physician assistant, Adrien!
Once ready, the neurotoxin will be injected at the target site. A single treatment session will typically last about 30 minutes and you will experience little to no downtime. With a professional neurotoxin treatment at Plastic Surgical Associates in Colorado, you should begin noticing results in a matter of days and continue to see the benefits for up to three to six months with Xeomin, and even longer with DAXXIFY™! To maintain your youthful appearance, we will recommend your own personalized injection treatment plan of two to four sessions per year.
Xeomin®
FDA approved and clinically proven for softening of dynamic lines*, Xeomin® is an industry standard when it comes to neurotoxins. Dynamic lines form when facial expressions are repeatedly made. Over time, as your skin ages, these repeated expressions cause lasting lines with repeated use of the same facial muscles. Xeomin® blocks the release of chemicals that cause these muscle contractions so lines are softened.
*Xeomin® (incobotulinumtoxinA by Merz Pharmaceuticals) is FDA-approved for the of adults with cervical dystonia or blepharospasm and/or moderate to severe dynamic lines between the eyebrows (glabella).
Efficacy and purity are always a concern of Dr. Duncan’s, and Xeomin®’s unique manufacturing process uses two filtration steps to remove unnecessary components that aren’t active in treatment. Do note that comparative studies evaluating relative risk management due to the presence or absence of these unnecessary components have not been performed but we try to be as conservative and safe as possible!
Daxxify is HERE!
We’re pleased to introduce DAXXIFY™–the only long-lasting, peptide-powered frown line treatment. We’re one of the first exclusive providers to offer this innovative treatment. Call our office today to book your appointment and visit DAXXIFY.com to learn more.
What is DAXXIFY™ and how is it different?
DAXXIFY™ is FDA approved to help smooth moderate to severe lines between the
brows. It is the only long-lasting frown line treatment powered by a peptide with
results that last on average 6 months and up to 9 months for some.*
Conventional injectables last 3 – 4 months and require up to 4 treatments a year to maintain results. Nearly 90% of patients say they wish results lasted longer. With DAXXIFY™ you can keep your frown lines smoother for a full year with as few as 2 treatments.*
*At least 50% of patients in clinical studies had no or minor frown lines 6 months after treatment. Between 3% and 5% of patients in clinical studies had no or minor frown lines 9 months after treatment. In other safety studies, between 5% and 17% of patients still had noticeable improvement 9 months later.
Why are peptides important?
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant. DAXXIFY™ is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
Is DAXXIFY™ well studied?
DAXXIFY™ was studied in the largest-ever clinical study conducted for a frown line treatment and included more than 2,400 people across different ages and skin types.
• There were no serious treatment-related side effects in clinical trials for
DAXXIFY™
• The active ingredient in DAXXIFY™ is botulinum toxin type A, an ingredient that has been used in frown line treatments for more than 20 years
• 96% of people treated with DAXXIFY™ were satisfied with their results†
†96% of patients reported they were satisfied on a 7-point scale when evaluated at 4 weeks in clinical studies.
What safety information do I need to know with regards to DAXXIFY™?
DaxibotulinumtoxinA-Ianm injection (DAXXIFY™) may cause serious side effects that can be life threatening (similar to any neurotoxin). Get medical help right away if you have any of these problems any time (hours to weeks) after injection of DAXXIFY™:
• Problems swallowing, speaking, or breathing due to weakening of associated muscles can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
• Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms that include loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly,
loss of bladder control, trouble breathing, and trouble swallowing.
Do not receive DAXXIFY™ if you are allergic to any of the ingredients in DAXXIFY™ (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®/BOTOX® Cosmetic), abobotulinumtoxinA (DYSPORT®), incobotulinumtoxinA (XEOMIN®) or prabotulinumtoxinA-xvfs (JEUVEAU®); or have a skin infection at the planned injection site.
DAXXIFY™ dosing units are not the same as, or comparable to, any other botulinum toxin product. Tell your healthcare provider about all your medical conditions, including any side effects from botulinum toxin products, including dry eye; breathing, swallowing, bleeding, or heart problems; plans to have surgery; weakness of forehead muscles; drooping eyelids; have had surgery on your face; are pregnant or breastfeeding or plan to become pregnant or breastfeed. Tell your healthcare provider about all the medicines you take, including prescription and over-the counter medicines, vitamins, and herbal supplements.
Using DAXXIFY™ with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your healthcare provider that you have received DAXXIFY™ in the past.
Especially tell your healthcare provider if you have received any other botulinum toxin product in the last 4 months or any in the past, and exactly which product you received (such as BOTOX®, BOTOX® Cosmetic, MYOBLOC®, DYSPORT®, XEOMIN®, or JEUVEAU®). DAXXIFY™ may cause serious side effects,
including allergic reactions (such as itching, rash, redness, swelling, wheezing, trouble breathing, or dizziness or feeling faint), heart problems (such as irregular heartbeat and heart attack), and eye problems (including dry eye, reduced blinking, and corneal problems). Tell your healthcare provider or get medical help right away if you experience a serious side effect. No serious adverse events of distant spread of toxin effect associated with dermatologic use of DAXXIFY™ have been reported in clinical studies at the dose of 40 Units for glabellar lines. The most common side effects of DAXXIFY™ include headache, eyelid drooping, and loss of ability to move the muscles in your face.
These are not all the possible side effects of DAXXIFY™. For more information, see the full Prescribing Information including Boxed Warning, and refer to the Medication Guide or talk with your doctor. To report side effects associated with DAXXIFY™, please call 1-877-373-8669. You may also report side
effects to the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
If you are interested in any of our available positions, please submit a resume and cover letter to acarpenter@drdianeduncan.com.
